1.10 Equilibrium constant Kc for homogeneous systems (Equilibrium A2)
Chemical kinetics (rates of reaction) is concerned with how fast reactions proceed.
Chemical equilibria are concerned with how far reactions proceed.
They are distinct branches of chemistry and must not be mixed up.
Reminder (click here AS Equilibrium)
 Chemical equilibrium: is the state reached when the concentrations of reactants and products remain constant over time in a reversible reaction.
Chemical equilibrium: is the state reached when the concentrations of reactants and products remain constant over time in a reversible reaction.
Reactant and product concentrations stabilise because the rate of the forward reaction equals the rate of the reverse reaction at equilibrium.
A homogeneous equilibrium has all reactant and product species in the same phase e.g. all gaseous or all in the same solution.
The equilibrium law and the equilibrium constant Kc
The equilibrium constant Kc is deduced by utilising the equation for a reversible reaction and experimentally measuring at least one [product] or [reactant] at dynamic equilibrium.
The concentration, in mol dm-3, of a species X involved in the expression for Kc is represented by [X]
Equilibrium Law: if the concentrations of all the substances present at dynamic equilibrium are raised to the power of the number of moles that they appear in the chemical equation, then the product of the concentrations of the products divided by the product of the concentrations of the reactants equals the equilibrium constant, Kc .
What affects Kc ?
The value of the equilibrium constant is not affected by either changes in concentration, pressure or addition of a catalyst. A catalyst speeds up both forward and backward reactions by equal amounts, hence it has no effect on the position of the equilibrium and just speeds up the rate at which dynamic equilibrium is established (where the rates of the forward and reverse reactions become equal).
However, equilibrium constants are temperature-dependent, so the temperature must be stated for a given Kc .
For example, Kc for decomposition of N2O4 increases from 4.64 x 10−3 at 25 °C to 1.53 at 127°C.
 If the forward reaction is endothermic, increasing temperature shifts the equilibrium to the right favouring the forward reaction, increasing Kc and increasing the yield of the product.
If the forward reaction is endothermic, increasing temperature shifts the equilibrium to the right favouring the forward reaction, increasing Kc and increasing the yield of the product.
If the forward reaction is exothermic, decreasing temperature shifts the equilibrium to the right favouring the forward reaction, increasing Kc and increasing the yield of the product.
The conditions applied to maximise yield in the Haber process typify these principles.
ONLY a change in temperature will alter the equilibrium constant Kc
Common misconceptions between Le Chatelier’s principle, equilibrium constant Kc and the rate of reaction constant (κ)
Le Chatelier’s principle states that, when a system at equilibrium is subjected to change (such as concentration, temperature, volume, or pressure) then the system readjusts itself (alters the position of its equilibrium) to oppose the change.
Remember, pressure change only affects gaseous equilibria and only then when there are an unequal number of moles of gas on either side of the chemical equation for the equilibrium.
EXPANDING UPON THIS, AND CONSIDERING THE EFFECT UPON A DYNAMIC EQUILIBRIUM SYSTEM:-
- Changes in concentration, volume or pressure will alter the position of the equilibrium (favouring the greater formation of either the product(s) or the reactant(s)), however, the value of Kc remains constant. The opposing alterations between reactant(s) and product(s) concentrations ensures Kc remains constant.
! BUT !
- Changes in temperature will alter the position of the equilibrium AND alter the value of Kc .
For example, increasing the temperature of an exothermic equilibrium reaction will decrease Kc , move the position of the equilibrium towards the reverse reaction (which is endothermic and will ‘absorb’ the heat) and decrease the formation of product.
Under standard conditions, the Van’t Hoff equation applies, which mathematically relates Kc to change in Temperature and enables the derivation of enthalpy and entropy.
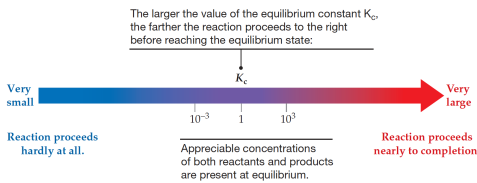
Using Kc to judge the extent of the reaction
In general :
If Kc > 103, products predominate over reactants: the reaction proceeds nearly to completion.
If Kc < 10−3 reactants predominate over products: the reaction hardly proceeds at all.
If Kc is between 10−3 to 103, appreciable concentrations of both reactants and products are present.