Born-Haber cycles
Enthalpy of hydration ΔH⦵hyd
Standard enthalpy change that occurs when 1 mol of gaseous ions are hydrated to form 1 mol hydrated ions in an infinitely dilute solution under standard conditions.
Na+(g) + aq → Na+(aq) ΔH⦵hyd =−390 kJ mol−1
Br−(g) + aq → Br−(aq) ΔH⦵hyd =−335 kJ mol−1
Cl−(g) + aq → Cl−(aq) ΔH⦵hyd =−364 kJ mol−1
Explaining trends in hydration of ions
1. Water is polarised, with each water molecule containing Hδ+ and Oδ−.
2. Na+ are attracted to Oδ− of the water molecule, which results in energy release as Na+ are hydrated.
Similarly, Cl− are attracted to Hδ+ of the water molecule, which results in energy release as Cl− are hydrated.
3. Moreover, because Cl− are smaller (so higher charge density) than Br−, they exhibit a stronger force of attraction to
Hδ+ of water molecule, so there is a greater energy release when Cl− are hydrated, hence, higher reported negative enthalpy of hydration.
Enthalpy of solution ΔH⦵solution
Standard enthalpy change that occurs when 1 mol of a solute is dissolved in a solvent to form an infinitely dilute solution under standard conditions.
NaCl(s) + aq → Na+(aq) + Cl−(aq) ΔH⦵solution =+13 kJ mol−1
KNO3(s) + aq → K+(aq) + NO3−(aq) ΔH⦵solution = +34.9 kJ mol–1 ΔS⦵= +117 J K–1 mol–1
Entropy change is positive because there is an increase in the number of particles (1 mol solid → 2 mol aqueous ions) and an increase in disorder. Considering dissolving KNO3(s) and applying ΔG⦵ =ΔH⦵ −TΔS⦵,
the temperature at which free-energy change, ΔG, for the process is zero occurs when ΔH⦵ = TΔS⦵.
So, T = ΔH⦵ /ΔS⦵ =34.9 ×1000/117 = 298.3 K.
Thus, at T>298.3K, ΔG⦵ <0, so there is spontaneous dissolving of KNO3.
Conversely, at T<298.3K, ΔG⦵ >0, so dissolving KNO3 is no longer spontaneous (no longer feasible).
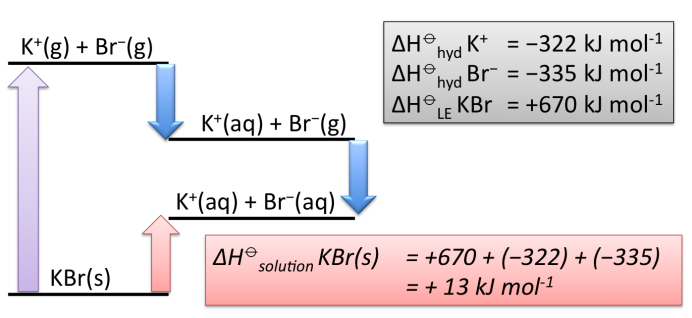
Calculating enthalpy of solution for ionic compounds using ΔH⦵L.E and ΔH⦵hyd
ΔH⦵solution=ΔH⦵dissociation L.E + Σ ΔH⦵hyd
Questions to check understanding of hydration and solution enthalpies
Q1. Given the following data, calculate the enthalpy of hydration of magnesium ions.
Mg2+(g) + aq → Mg2+(aq) ΔH⦵hyd = hyd
Cl−(g) + aq → Cl−(aq) ΔH⦵hyd = −364 kJ mol−1
MgCl2(s) → Mg2+(g) + 2Cl−(g) ΔH⦵L.E = +2493 kJ mol−1
MgCl2(s) + aq → Mg2+(aq) + 2Cl−(aq) ΔH⦵solution = −155 kJ mol−1
ΔH⦵solution= ΔH⦵dissociation L.E + Σ ΔH⦵hyd
−155 = +2493 +(ΔH⦵hyd Mg2++ 2(−364))
−155 = +2493 +(ΔH⦵hyd Mg2+ −728)
ΔH⦵hyd Mg2+ = −1920 kJ mol−1
Q2. The enthalpy of solution for silver fluoride in water is –20 kJ mol–1. The hydration enthalpy for silver ions is –464 kJ mol–1 and fluoride ions are −506 kJ mol–1.
i) Calculate a value for the lattice enthalpy of dissociation of silver fluoride.
ii) Suggest why the entropy change for dissolving silver fluoride in water has a positive value.
iii) Explain why the dissolving of silver fluoride in water is always a spontaneous process.
i) ΔH⦵solution= ΔH⦵dissociation L.E + Σ ΔH⦵hyd
−20 =ΔH⦵L.E +( −464 + (−506)) So, ΔH⦵L.E silver fluoride = +950 kJ mol−1
ii) There is an increase in disorder and number of particles (1 mole solid → 2 moles of aqueous ions)
iii) For a reaction to always be spontaneous ΔG must be negative from ΔG = ΔH − TΔS. In relation to dissolving silver fluoride in water, ΔH is negative and ΔS is positive, so for any value of T, ΔG is always a negative value.
Q3. Use these data to calculate a value for the enthalpy of solution for CaF2
Ca2+(g) + aq → Ca2+(aq) ΔH⦵hyd = −1650 kJ mol−1
F−(g) + aq → F−(aq) ΔH⦵hyd = −506 kJ mol−1
CaF2(s) → Ca2+(g) + 2F−(g) ΔH⦵L.E = +2611 kJ mol−1
CaF2(s) + aq → Ca2+(aq) + 2F−(aq) ΔH⦵solution = ?
ΔH⦵solution=ΔH⦵dissociation L.E + Σ ΔH⦵hyd = +2611 + (−1650 + 2(−506)) = −51 kJ mol−1
Q4. Calcium chloride dissolves in water. After a certain amount has dissolved, a saturated solution is formed and the following equilibrium is established: CaCl2(s) ⇌ Ca2+(aq) + 2Cl−(aq)
i) Using the data below, calculate the enthalpy change for this reaction.
ii) Predict whether raising the temperature will increase, decrease or have no effect on the amount of solid calcium chloride that can dissolve in a fixed mass of water.
Enthalpy of lattice dissociation for calcium chloride +2237 kJ mol−1
Enthalpy of hydration for Cl– ions −364 kJ mol−1
Enthalpy of hydration for Ca2+ ions −1650 kJ mol−1
i) ΔH⦵solution=ΔH⦵dissociation L.E + Σ ΔH⦵hyd = +2237 + (−1650 + 2(−364)) = −141 kJ mol−1
ii) Dissolving CaCl2(s) is an exothermic reaction (ΔH⦵solution −141 kJ mol−1). Increasing the temperature will move the position of the equilibrium to the left to oppose the increase in temperature, so less CaCl2(s) will dissolve.